Background and aims
Chimeric Antigen Receptor (CAR) T-cell therapies for relapsed-refractory Pediatric B-cell Acute Lymphoblastic Leukemia (r/r-B-ALL) show remarkable success but remain prohibitively expensive. We developed and reported a Humanized CD19-directed novel CAR T-cell- HCAR19 (now -Actalycabtagene autoleucel- Actaly-cel™) with robust activity and low toxicity in pre-clinical studies. This led to regulatory approval for the first CART-cell trial in India as a Pilot Phase-1 study (CTRI/2021/05/033348), and it's encouraging safety profile and responses led to approval for a roll-on Phase 1b/2 Trial (CTRI/2023/03/050689) to monitor safety, identify Phase-2 Dose (P2D) and assess efficacy. Trial design permitted pooling of data of both trials for all objectives. We report here the Phase-1b analysis of Actaly-cel in r/r-B-ALL in Pediatric, Adolescents and Young Adults 3-25 years of age.
Methods
Patients with r/r B-ALL not in remission after 2 or more lines of therapy ineligible for Allogenic-Stem Cell Transplant (Allo-SCT) were included if no CD19-negative blasts population were detected on flow-cytometry records. Those with isolated extra-medullary disease, active neurological involvement/ sequelae, genetic syndromes/prior malignancy, or prior CD19-directed therapy were excluded. Bridging therapy was at physician discretion. Lymphodepletion was with Fludarabine (30mg/m 2) x 3-days and cyclophosphamide (500 mg/m 2) x 2-days. There were 3 Target Doses, TD-1, 2 & 3 CART-cells at 1 x10 6, 3-5 x10 6 and 10-15 x10 6/kg recipient weight respectively. Escalation and de-escalation followed 3+3 design with Coherence-guided dose-decision for bivariates- efficacy and toxicity, allowing all patients to be evaluable. Primary end-point was Overall Response Rates (ORR) at Day-30 on bone-marrow flow-cytometry. Patients were monitored for toxicities, in-vivo dynamics of Actaly-cel, and cytokine profiles.
Results
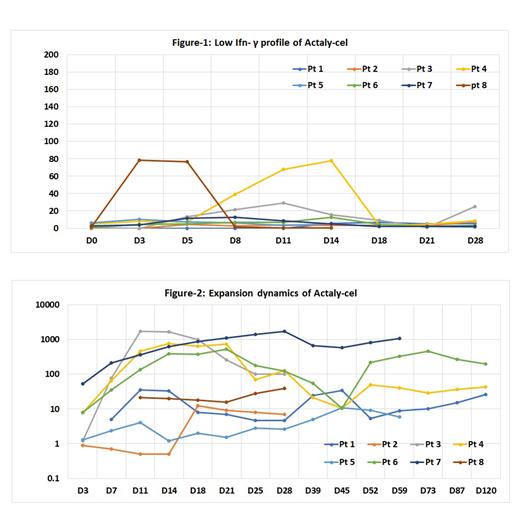
Of 12 patients enrolled Actaly-cel was infused in 9 at time of analysis as 2 were withdrawn prior to apheresis (1-progression, 1-withdrawl of consent) while 1 is awaiting infusion. Three patients were assigned TD-1 and 6, TD-2. In TD-2 group, 1 received lower dose (2.13x10 6/kg) due to obesity and weight gain from severe-Covid19 prior to infusion. Median age was 16-yrs (range:8-24), M:F-3:2, median prior lines of therapy were 3 (range:2-5), 6 had high-risk cytogenetics, and median pre-infusion bone-marrow blasts were 19.5%(range:0-88%). Eight patients were evaluable for response and toxicities. All had Grade 3 leukopenia and thrombocytopenia. Peak cytokine release syndrome (CRS) was Grade-3 in 1(12.5%), Grade-2 in 2(25%), and Grade-1 in 5(62.5%). Those with ≥ Grade-2 CRS received tocilizumab with full recovery. Only 1 patient had Immune effector cell associated neurotoxicity syndrome (ICANS) peak-Grade 3, and was also the only one with Grade-3 CRS and had severe-Covid19 prior to infusion. ICANS resolved with steroids in 4 weeks, but recurred in the second month post-infusion, this time associated with leukemia-progression. Toxicities corroborated with IL-6 (median peak: 25pg/ml, range:2.8-3604), but not with Ifn-γ, which remained low (median peak:6.25pg/ml, range:0-68.1) (Fig-1). ORR was 87.5% (1 no-response-NR), 3 Morphological Remission with MRD-positive (Partial Response-PR), and 4 MRD-negative (Complete Response-CR). Responses correlated with Actaly-cel expansion dynamics (Fig-2) the only non-response being in a patient with poor expansion. At median follow-up of 3 months (range:1-15) 3 patients with no or partial Day-30 response expired from progression within few weeks. All 4 with CR had B-cell aplasia needing intravenous immunoglobulin. Two of them underwent subsequent Allo-SCT, 1 relapsed post-transplant and died from disease progression 15 months later, while the other is alive in remission at 15 months. Two patients remain in CR at 3 and 12 months without further therapy, while 1 with PR is on follow-up. Patients receiving TD-2 had ORR of 100% (CR-3, PR-1) with peak toxicity of Grade-2 CRS in only 1.
Conclusion
In a Phase-1/1b trial, Actaly-cel had good safety profile with low toxicity and robust activity resulting in high ORR that improved at higher doses. In-vivo dynamics and toxicity profile of Actaly-cel showed robust activity and low cytokines corroborating with pre-clinical studies. A median dose of 5x10 6/kg Actaly-cel is proposed as the P2D.
Disclosures
Jain:Intas Pharmaceuticals: Research Funding; Zydus Pharmaceuticals: Research Funding; ImmunoACT: Research Funding. Karulkar:Immunoadoptive Cell Therapy Private Limited: Current Employment, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Shah:Immunoadoptive cell therapy private: Current Employment, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Firfiray:Immunoadoptive Cell Therapy Private Limited: Current Employment, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Thorat:Immunoadoptive Cell Therapy Private Limited: Consultancy. Shah:Immunoadoptive Cell Therapy Private Limited: Consultancy, Other: Scientific Advisory Board; CARGO: Consultancy; VOR: Consultancy, Research Funding; Lentigen: Research Funding. Purwar:Immunoadoptive Cell Therapy Private Limited: Current Employment, Current equity holder in private company, Patents & Royalties.